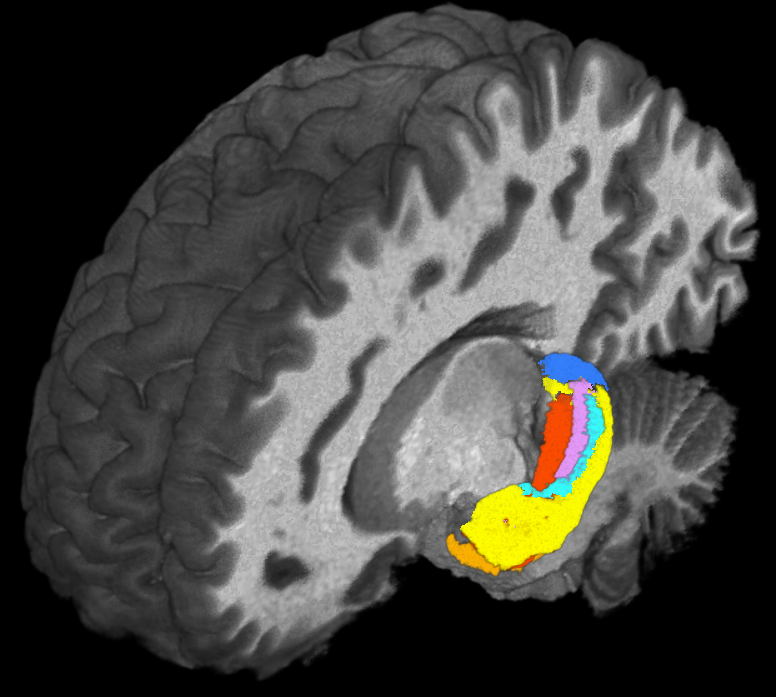
Using ultra-high field magnetic resonance imaging (MRI) to map the brains of people with Down syndrome (DS), researchers from Case Western Reserve University, Cleveland Clinic, University Hospitals and other institutions detected subtle differences in the structure and function of the hippocampus—a region of the brain tied to memory and learning.
Such detailed mapping, made possible by the high-powered MRI, is significant because it allowed the research team to better understand how each subregion of the hippocampus in people with DS is functionally connected to other parts of the brain.

“The ultimate goal of this approach is to have an objective technique to complement neuropsychological assessments to measure the functional skills of those with DS,” said Alberto Costa, professor of pediatrics and psychiatry at the Case Western Reserve University School of Medicine and the study’s senior author.
Their study was recently published in Brain Communications.
Down syndrome is a genetic condition typically caused by having an extra copy of chromosome 21. The extra chromosome changes how a baby’s body and brain develop, which can cause mental and physical challenges throughout the person’s life.
The intellectual and developmental disabilities of individuals with DS are typically generalized. In other words, although abilities can range widely among people with DS, different types of cognitive skills are usually affected in a similar way in the same person. However, for a given person with DS, cognitive abilities that are heavily dependent on the hippocampus are especially affected.
“That’s why we focused on this structure deep inside the brain that is responsible for functions such as forming memories of episodes in one’s life and spatial memory,” Costa said.
MRI scanners at ultra-high magnetic field strengths are increasingly available for human research, allowing neuroscientists to map the brain at higher resolution without losing image clarity.
Taking advantage of the increased resolution afforded by high-powered MRI, the researchers performed the first in-vivo comparison of volumes of different anatomical segments of the hippocampus between people with DS and “control” individuals of the same age and sex without DS.
“The gains in sensitivity and image resolution achievable with ultra-high field MRI provide levels of detail and accuracy that have not previously been attainable in studies of live, non-sedated individuals with Down syndrome,” said Katherine Koenig, an assistant professor of radiology at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University and the study’s first author.
“We also found significant relationships between the size of subregions of the hippocampus and cognitive measures,” Costa said. “Although more work will be necessary to validate some of our findings, these results support the investigation of specific MRI measures as potential markers to study drug efficacy for possibly enhancing cognitive function in persons with Down syndrome.”
The research was supported by: the Alana USA Foundation, the Alzheimer’s Association, the Cleveland Alzheimer’s Disease Research Center, the National Institute on Aging, the Cleveland-based Awakening Angels Foundation and the National Institute on Aging.
The research team included: Z. Irene Wang, director of research at Cleveland Clinic Epilepsy Center; and James Leverenz, director of the Cleveland Lou Ruvo Center for Brain Health – Neurological Institute and Jane and Lee Seidman Endowed Chair for Advanced Neurological Research and Education at Cleveland Clinic; Stephen Ruedrich, the L. Douglas Lenkoski Professor in the Department of Psychiatry at Case Western Reserve and University Hospitals Cleveland Medical Center; Se-Hong Oh, an assistant professor in the Department of Biomedical Engineering at the Hankuk University of Foreign Studies; Melissa Stasko, a research assistant in Costa’s lab; Elizabeth Roth, a research assistant in the Department of Pediatrics at the School of Medicine; H. Gerry Taylor, a professor of pediatrics at the Center for Biobehavioral Health, Abigail Wexner Research Institute at Nationwide Children’s Hospital and The Ohio State University.
For more information, contact Bill Lubinger at william.lubinger@case.edu.
This article was originally published April 29, 2021.