Case Western Reserve University licenses new sensor technology to company pursuing fast-track FDA approval
Case Western Reserve University researchers have developed a portable sensor that can assess the clotting ability of a person’s blood 95 times faster than current methods—using only a single drop of blood.
Even better, the device provides more information about the blood than existing approaches.
Rapid and accurate assessments are essential to ensuring that patients prone to blood clots—as well as those who have difficulty clotting—receive care appropriate to their conditions.
 Last week, XaTek, a new Cleveland-based company, licensed the technology for the device—called ClotChip—with a goal of bringing it to market within the next three years. Case Western Reserve’s Technology Transfer Office negotiated the agreement between the university and the company.
Last week, XaTek, a new Cleveland-based company, licensed the technology for the device—called ClotChip—with a goal of bringing it to market within the next three years. Case Western Reserve’s Technology Transfer Office negotiated the agreement between the university and the company.
“ClotChip is designed to minimize the time and effort for blood-sample preparation. [It can] be used at the doctor’s office or other points of care for patients on anticoagulation therapy, antiplatelet therapy or who have suffered a traumatic injury causing bleeding,” said Pedram Mohseni, professor of electrical engineering and computer science (EECS) at Case Western Reserve, who led the development of ClotChip with Michael Suster, senior research associate in the EECS department.
Existing measures typically require patients to visits laboratories where expert technicians administer tests, an approach that typically is time-consuming and expensive. While a few methods exist to allow on-site testing, to date they have not proved nearly as precise as laboratory-based versions.
In preliminary tests, however, Case Western Reserve’s technology provided results in 15 minutes, as compared to conventional measures that can take a day or longer to yield results. ClotChip also provided more information about the coagulation process, including the effects of a new class of drugs called target-specific oral anticoagulants, or TSOACs.
TSOAC drugs block clots from forming in a different way than warfarin (most commonly known by the brand name Coumadin), which had dominated the market for decades. Warfarin, however, can interact negatively with several medications and foods and also requires frequent blood tests to monitor the drug’s effects.
The new medications, including rivaroxaban (Xarelto) and apixaban (Eliquis), have been marketed extensively as far more convenient alternatives. To date, however, the U.S. Food and Drug Administration (FDA) has not approved a device to determine the impact of the new drugs.
With the market share of TSOACs growing rapidly, “there’s a huge opportunity and need,” said John Zak, president and CEO of XaTek. “There’s no readily available point-of-care, cost-effective and accurate way to monitor these drugs.”
Zak, who earned his medical degree at Case Western Reserve, has been active in the region’s health entrepreneurship sector for more than a decade. The CEO said that the company hopes to complete a pilot clinical study and data analysis of ClotChip use on 200 patients at the Louis Stokes Cleveland VA Medical Center by the third quarter of 2017.
If the device proves effective in that initial evaluation, the company would seek to launch a full clinical trial within the following two years; from there, XaTek would seek FDA approval.
Among the founding directors of XaTek are John Nottingham and John Spirk, co-presidents of Nottingham Spirk Inc., the Cleveland-based business innovation and product design firm.
To monitor clotting, ClotChip uses an electrical technique called miniaturized dielectric spectroscopy, an approach that Mohseni, Suster and their team began developing six years ago. In essence, the technique applies an external electric field to the drop of blood, then quantitatively measures how the blood affects that field. The measurements reflect the ability of the blood to clot.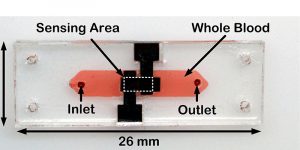
Two years ago, the engineering researchers began collaborating with Evi Stavrou, an assistant professor of hematology and oncology at Case Western Reserve School of Medicine. Together they found that ClotChip’s sensitivity to the blood coagulation process made it an appealing option for point-of-care testing.
Earlier this year, the engineering researchers shared preliminary findings in a paper that was part of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. On Monday, Stavrou will present the technology at the American Society of Hematology annual meeting; her abstract represents the first peer-reviewed dissemination of tests involving samples for more than 30 volunteers.
“Our device gives you different information—and more information—than other devices out there,” Stavrou said. “The sensitivity and discriminatory ability of the device, when compared to standard coagulation tests, is what excites me very much.”
Because the device works so quickly, emergency responders could use it on site to determine whether a patient in trauma is on one of the blood thinner medications. Such critical information also could be invaluable to medics in wartime.
The research was led by Advanced Platform Technology (APT) Research Center investigators Mohseni, Suster and Stavrou, who is also a hematologist at the Cleveland VA and will lead the clinical trials.
They teamed with doctoral students Debnath Maji, Erdem Kucukal and Ujjal Didar Singh Sekhon; recent PhD graduate Mehran Bakhshiani; Umut Gurkan, assistant professor of mechanical and aerospace engineering and APT Center investigator; and Anirban Sen Gupta, associate professor of biomedical engineering.
Funding for the research was provided by the Case-Coulter Translational Research Partnership, the APT Center at the Cleveland VA and the Case School of Engineering’s San Diego-based wireless health program.


